
Using AI to Decarbonize
the Chemicals Industry
The chemicals industry, a cornerstone of modern life, faces a critical challenge: balancing industrial growth with environmental responsibility. By harnessing AI and biocatalysis, we can pave the way for a more sustainable future. In recent years, expanding biological data and advances in computational techniques have given rise to AI algorithms capable of everything from designing new proteins from scratch to predicting diseases from any possible mutation. These tools are already being broadly applied across drug development with their potential impact soon to emerge. Yet there remains an underserved application: their use across industrial biotechnology as a means to help clean up sectors that have polluted our communities and planet. The time has come to build circular economies and a better world for our children.
For more than 75 years production of chemicals has increased to now exceed 1T pounds annually in the US alone. The vast majority of these chemical products are derived from oil & gas feedstocks using catalytic processes that are usually energy intensive and often generate toxic byproducts. Together, this production represents part of an ~$6T industry. But that industry faces increasing pressure from both consumers and regulatory agencies to reduce waste and increase circularity/sustainability. Together, leading chemicals producers are investing ~$7B each year in support of these mandates.
Across industries there exist diverse process waste streams from CO2 or other gasses to biomass or plastics. Often, these are either simply vented into the atmosphere, buried in a landfill, or worse yet, finding their way into the natural environment where they can wreak havoc on plant, animal, and human populations. In the face of population growth, consumer demand, and sustainability goals, the pressure to decarbonize the chemicals industry has never been higher.
Over the course of at least the last decade, thanks in part to investments across the pharmaceutical industry, the cost of protein manufacturing has been significantly reduced. This has lowered both small scale costs for early design-build-test-learn cycles and for scaled commercial operations. At the same time computational tools have made striking advances in predictive power across biological applications thanks in part to growing genomic and proteomic datasets. Together, these have led to expanded abstraction and services offerings across the biotechnology sector which facilitates greater outsourcing and capital efficiency.
On a similar timetable, we’ve seen the advance of computational approaches in biology grow from flux balance analysis, molecular dynamics, and physics-based models to neural networks, large language models, and de novo design. Even more recently, researchers including Levin et alhave begun to apply classically chemical approaches like retrosynthesis to biology using AI (neural networks). Such efforts allow us to escape the confines of evolved metabolisms and the stitching together of heterologous pathways to achieve industrial manufacturing goals. Over the eons, natural biology evolved for growth within ecological niches, not optimal production of chemical products of interest. Thus, we set the stage for the potential to use enzymatic retrosynthesis search to map novel pathways/processes for chemical manufacturing. This unconstrained biotransformation space, loosely defined as all possible enzymatic catalysis multiplied by all known chemical intermediates, is not only non-navigable by the human mind but wildly undersampled to date.
Given AI algorithms capable of navigating unconstrained biotransformation space, we stand on the precipice of dramatic improvements in energetic efficiency and theoretical yield for biology. Doing so will unlock market opportunities particularly in the context of waste-to-value economies. Low-to-negative feedstock costs combined with optimized biotechnology are the keys to decarbonization in the chemicals industry. From initial screening through commercialization, individual enzymes and aggregate biotransformation pathways can be produced using cell-free systems to create the simplest and most efficient reaction conditions. Along the way, process parameters can be optimized depending on the features of the feedstock and product at hand. All of this engineering contributes to meeting commercial thresholds set by continuous techno-economic modeling to ensure we’re meeting the pricing realities of the market.
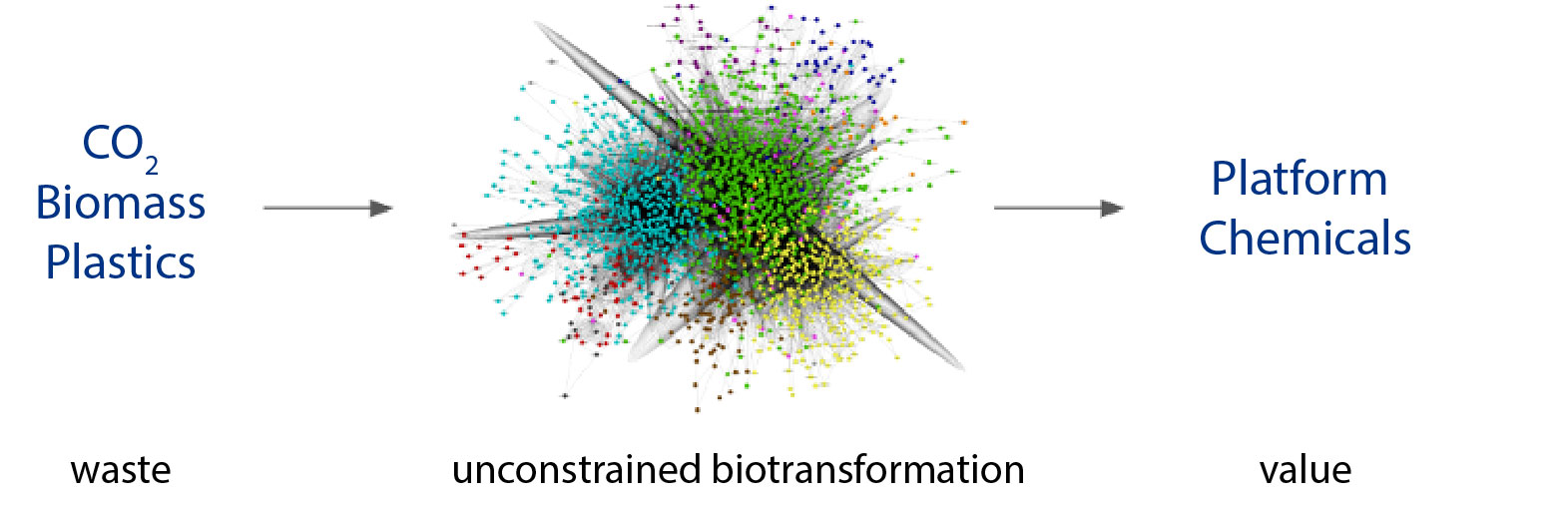
First generation synthetic biology companies were often focused on biofuels production thanks in part to the giant market size carrot at the end of the stick, but as they moved towards commercialization the practicalities of commodity unit economics in the backdrop of contemporary biology tools became untenable. As the pendulum swung for the second generation, most focused on higher value ingredient products such as flavors and fragrances or cosmetics. While certainly easing unit economic constraints, ingredients faced a rather high spec threshold which challenged drop-in adoption and by extension revenue growth. Based on the aforementioned advances in technology as well as others, there exists an opportunity to pursue the goldilocks marketplace of ‘platform chemicals’. These are chemical products which tend to be manufactured in excess of 1M tonnes per year, sold in the $2-10/kg range, and utilized across industry verticals. Exemplary products might have uses ranging from pharmaceuticals, food, and cosmetics to de-icing agents, plastics, surfactants and lubricants. Their global markets are typically estimated in the >$1-5B range annually with domestic production typically exceeding millions of tonnes. The existing marketplaces for such chemicals are dominated by large multinational chemical companies using oil and gas component feedstocks and manufacturing processes that haven’t seen innovation in decades.
Across these chemical markets the opportunity for disruption exists. Successful AI-driven identification of waste (green dots) to product (red dot) pathways and process optimization will represent a disruptive entrant to these marketplaces based on both existing producers’ sustainability objectives and existing consumers’ demand for cost parity at the same time. Some of this opportunity stems from differences in feedstock cost: whereas bulk CO2 is often quoted at roughly $50/tonne, other sustainables like glycerol and sugars range from $250-750/tonne (used by microbial processes for green chemicals), and unsustainable feedstocks quoting at ~$1500/tonne. Given the value proposition for sustainability, particularly among food and cosmetic customers, we believe achieving price parity should be sufficient to drive market adoption in this class of products.
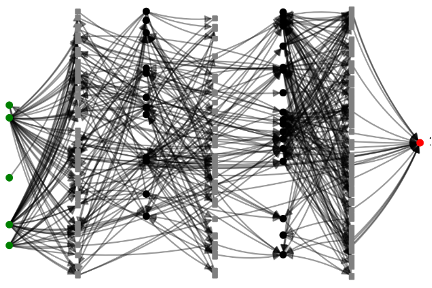
Interestingly, the problem of waste generation/remediation and sustainable chemical production is often experienced by the same companies. Major chemical companies suffer from multiple and diverse waste streams that they are eager, nay obliged, to see mitigated and are simultaneously interested in the ability to develop new more sustainable processes for manufacturing. De novo designed biocatalytic pathways and cell-free reactor systems represent an opportunity for them to potentially do both at once. Elsewhere, product end users lament current supply chain dynamics and desire (sometimes as an extension of their own customers’ desires) more sustainable sourcing. For oil-derived platform chemicals, indeed there are many potential end users who may not have the internal or customer imperative for sustainability but whose adoption thereof will benefit society.
Assuming a $4/kg unit price for target product, then a first $1M in revenue could be realized upon production of 250 tonnes. This scale might be achieved initially in a pilot facility with reactors on the scale of 5-10k liters over a longer time frame (and at lower margins) or in a higher capex facility similar to that modeled by Singh et al wherein their assumptions allow for achieving such volumes on a weekly basis. In either scale setting, AI algorithms are already being developed to integrate diverse process parameter data streams to help optimize product yield/productivity and thus margins. Prior to fundraising and capital investment in production facilities, target products should be thoroughly vetted through primary market research with prospective customers to secure letters of intent or perhaps even off-take agreements if product samples are available through benchtop validation. Early adopters at even higher price points can be initially serviced by pilot facility production to begin generating revenue as production ramps up.

When it comes to sustainably producing specialty chemicals, there has been steady progress achieved through fits and starts. A number of innovation companies have arisen with their own attempts to address the decarbonization challenge. One of the most successful of these is LanzaTech, whose use of CO2 waste streams for the microbial production of ethanol has allowed them to scale a very successful business in a tough commodity market. Supported in part by millions of dollars from the Department of Energy and several industrial partnerships, they have successfully scaled up across multiple sites.
Others addressing sustainable chemical production have traditionally focused on dextrose as a feedstock since it is readily assimilated as a carbon source by most microbes. In general, when we examine the cost of even a simple single-step biocatalysis we find that a significant proportion of the production cost is linked to feedstock. While costs for microbial fermentation are almost certainly higher and theoretical yields lower, we can appreciate that starting with a feedstock of significant cost is inhibiting competitive economics below ~$10/kg. Compared to those using sugar, opportunities exist for those leveraging low-to-negative cost waste feedstocks and future optimization of biotransformation processes using multi-step pathways to maximize theoretical yields and thus margins while simplifying scale-up using cell-free processes.
Irrespective of advances in computational biotechnology and synthetic biology, the future catalytic potential/efficiency of AI-predicted, non-natural enzyme/substrate pairings will be foundational to success or failure in these approaches. As Bar-Even et al described years ago, nature tends to evolve only moderately efficient enzymes. And while our practitioners have decades of experience focusing and improving that efficiency using classical enzyme engineering approaches, some of the latest AI tools like LigandMPNN have improved effectiveness.
For each specific feedstock-to-product biotransformation, detailed techno-economic modeling initially anchored in assumptions can help set benchtop bioengineering goals and process optimization on the path to commercialization. Waste feedstocks of all kinds at varying price points are foundational to that modeling but also geographically fragmented. Future success in this regime will depend not only on securing product demand but also on aligning that demand with regional feedstock supply and tightening supply chain logistics.
In the not too distant future, biology may indeed eat everything, but we need to help it make sure that, at least for this meal, its eyes aren’t bigger than its stomach.
– Guest Blog Post by Christopher M. Pirie, PhD
Dr. Christopher Pirie is a biotechnology executive and serial entrepreneur. He has previously served as co-founder and Chief Operating Officer of HDT Bio where he helped to secure and service >$300M in federal grants/contracts, commercial partnership agreements around the world, and equity financing. Before that he was co-founder and CEO of Virvio, a biopharmaceutical company spun out of the Institute for Protein Design and focused on designer mini-proteins for respiratory diseases like influenza. He also co-founded Manus Bio, a synthetic biology company developing sustainable chemical manufacturing methods where he built and ran the enzyme engineering team while leading intellectual property development.
Dr. Pirie also coaches entrepreneurs around the world in collaboration with MIT Sloan Business School and has mentored early-stage startups through the Buerk Center for Entrepreneurship at UW. He received his PhD in Biological Engineering from MIT, focused on protein engineering, and a BS in Bioengineering from UW. When he’s not working, he is an avid soccer player and occasional sailor.